pd electron configuration|electron configuration worksheet : Cebu Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit pa Investing is a gamble, just like everything else. Investing in a globally diversified index fund is widely considered a prudent investment. On the stock market investing spectrum, this is about as far away from the ‘gambling side’ as you can get. And yet it is still a ‘gamble’. For example, you are choosing to invest in stocks over .
PH0 · pd+2 electron configuration
PH1 · full electron configuration of platinum
PH2 · electron configuration worksheet
PH3 · electron configuration periodic table
PH4 · electron configuration for every element
PH5 · electron configuration chart pdf
PH6 · electron configuration chart
PH7 · electron configuration calculator
PH8 · Iba pa
Bern old town. Bern is the capital city of Switzerland and is nestled in the heart of the country, between Geneva and Zurich.Bern’s location is great for exploring the rest of the country as it is a short train .
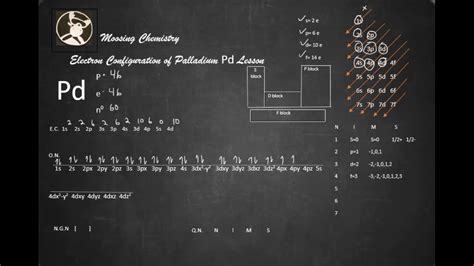
pd electron configuration*******The ground state electron configuration of palladium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10. This electron configuration shows that the d-orbital has a total of ten electrons. Therefore, the valence electronsof palladium are ten. The elements that form bonds by donating electrons are called cation. . Tingnan ang higit paThe total number of electrons in palladium is forty-six. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in palladium in specific rules in different . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit pa March 23, 2023. Electron configuration chart of all Elements . The answer is 46P d:1s2,2s2,2p6,3s2,3p6,4s2,3d10,4p6,5s0,4d10. The explanation is based on the Aufbau principle and the experimentally observed electron .
Palladium belongs to group 10 in the periodic table, but the configuration in the outermost electrons is in accordance with Hund's rule. Electrons that by the Madelung rule would be expected to occupy the 5s instead fill the 4d orbitals, as it is more energetically favorable to have a completely filled 4d shell instead of the 5s 4d configuration. This 5s configuration, unique in period 5, makes palladium the heaviest element having only on.Electron configuration of palladium. The Electron configuration of palladium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10. The chemical element that belongs to the periodic .Palladium is named after the asteroid Pallas, in turn named after the Greek goddess of wisdom, Pallas. Allotropes. Pd. Palladium. 46. 106.42. Glossary. GroupA vertical column .
Electron configuration of Palladium is [Kr] 4d10. Possible oxidation states are +2,4. Electron Configuration. The periodic table is a tabular display of the chemical .pd electron configuration What is The Electron Configuration of Pd? The Palladium element has the 46 electrons you can refer to the periodic table to calculate that and we further know about the S orbital, which can only retain the . The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter. For example; the electron configuration of palladium is [Kr] . Electronic Configuration of Elements. Page ID. Chung (Peter) Chieh. University of Waterloo. Learning Objectives. Explain the rules for filling electrons in atomic orbitals -- Pauli exclusion principle and .Electronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. Electronic configuration of Palladium (Pd): Palladium is a d-block element having atomic number 46. The group number and period number of Palladium are 10 and 5 respectively.
Hund's Rule. Hund's rule suggests that electrons prefer parallel spins in separate orbitals of subshells. This rule guides us in assigning electrons to different states in each sub-shell of the atomic .
Sarah Faizi (University of California Davis) 2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is . In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .
The Aufbau principle predicts that the 4s orbital is always filled before the 3d orbitals, but this is actually not true for most elements!From Sc on, the 3d orbitals are actually lower in .
Palladium is a chemical element of the periodic table with chemical symbol Pd and atomic number 46 with an atomic weight of 106.421 u and is classed as a transition metal. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 2: 4p 6: 4d 10: Electrons per shell: 2, 8, 18, 18: Valence electrons : 10: Valency electrons : 4:
The Palladium element has the 46 electrons you can refer to the periodic table to calculate that and we further know about the S orbital, which can only retain the maximum 2 number of electrons. Further P can hold 6, d can hold 10 and the f can at last hold 14. With this equation the electron configuration becomes as 1s 2 2s 2 2p 6 3s 2 .
Its electron configuration is. He: 1s2 He: 1 s 2. The three electrons for Li are arranged in the 1s subshell (two electrons) and the 2s subshell (one electron). The electron configuration of Li is. Li: 1s22s1 Li: 1 s 2 2 s 1. Be has four electrons, two in the 1s subshell and two in the 2s subshell.
pd electron configuration electron configuration worksheet An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully-filled whenever possible. . Pd: 2: 2 6: 2 6 10: 2 6 10: 0* . Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus (element 15) as an example, the concise form is [Ne] 3s 2 3p 3.electron configuration worksheet The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number . Electron Configuration Palladium The element Palladium is an exception to the standard diagonal rules.Palladium has 46 electrons and does not have a 5s orbit.Orbital diagram. Palladium electron configuration. Pd (Palladium) is an element with position number 46 in the periodic table. Located in the V period. Melting point: 1552 ℃. Density: 12.02 g/cm 3 . The order of filling the orbitals with electrons in the Pd atom is an exception to the rule. Expected electronic configuration 1s2 2s2 2p6 3s2 .Palladium, symbolized by Pd, is a silvery white metal that possesses unique electron configurations and atomic structures. In this section, we will delve into the electron configuration of palladium and explore its atomic properties. Key Takeaways: The electron configuration of palladium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s0 4d10. Now, The electron configuration of Pd is: [Kr] 4d 10 and The electron configuration of Pd 2+ is: [Kr] 4d 8. So, in this most common oxidation state of palladium (Pd 2+), if we see the electron configuration, then it possesses incomplete d-orbitals.

Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from .
If you have Telegram, you can view and join TEAM LAPAGAN right away. right away.
pd electron configuration|electron configuration worksheet